Description
|
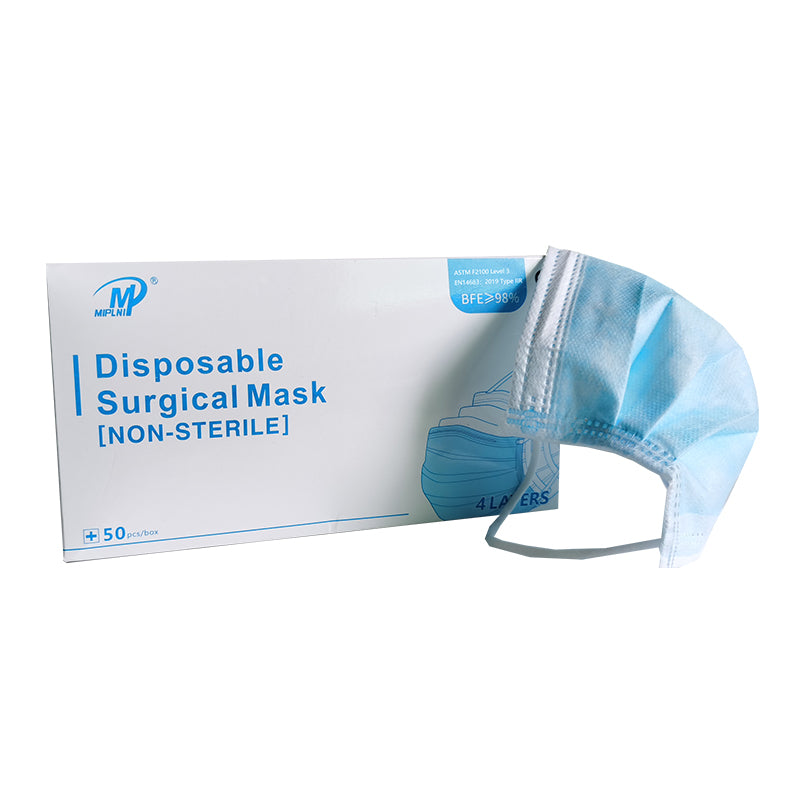
4 Layers Surgical mask(Not Sterile) Instruction for Use [ Storage conditions ) The product shall be stored in a well ventilated room with relative humidity no more than 80% and no corrosive substances. CE ( Product Name] Surgical mask(Not Sterile) Standard: ASTM F2100 Level 3,EN14683:2019 Type lIR ( Mode) YK-244 [Type specification) Flat ear hook type ( Size) 17.5x9.5cm [Structural composition) This product consists of cloth, nose clip and mask belt. The mask cloth and mask belt are made of nonwovens (the ear hanging mask belt is made of elastic belt), the nose clip is made of bendable plastic material, without ethylene oxide sterilization ( Intended use) The disposable surgical face mask is intended to be worn to protect both the patient and healthcare personnel from transfer of microorganisms, body fluids and particulate material. (It can not be used in places with strict microbiological control requirements such as intensive care room and operating room) ( Contraindications] It is forbidden for those who are allergic to product materials ( Directions for use) Open the package, take out the mask, straighten the nose bridge card, press the nose bridge card on it, put on the mask, adjust the nose bridge card along the nose bridge with the fingertips of both hands, from the middle to both sides, slowly press inward until it is close to the nose bridge and face. ( Precautions) 1. Read the manual carefully before use. 2. Single use only. 3. Discard the used product as medical waste. 4. It is forbidden to use the product if the package seals disconnected, damaged or beyond the validity period of the product. [ Expiry) 2years ( Symbol Description ) Caution, Indicates the need for the user to consult the A instructions for use for important cautionary information, such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. Authorized representative in the European Community, Indicates |EC | REP the Authorized representative in the European Community. CE Mark: conforms to essential requirements of the Medical CE Device Regulation 2017/745(EU). wl Date of manufacture, Indicates the date when the medical device was manufactured. Manufacturer, Indicates the medical device manufacturer, as defined in Medical Device Regulation 2017/745(EU). Use-by date, Indicates the date after which the medical device is not to be used. Do not re-use, indicates a medical device that is intended for one O use, or for use on a single patient during a single procedure. Do not use if package is damaged, indicates a medical device that W should not be used if the package has been damaged or opened. LOT Batch code, Indicates the manufacturer's batch code so that the batch or lot can be identified. * Keep away from sunlight, indicates a medical device that needs protection from light sources. * Keep dry, indicates a medical device that needs to be protected from moisture.
|